Abstract
Activating mutations in the Fms-like tyrosine kinase 3 gene (FLT3) are the most frequently observed molecular abnormalities in AML, which lead to overexpression or constitutive activation of the tyrosine kinase. While several mechanisms of resistance to FLT3 inhibitors have been identified, commonly failure is associated with lack of complete and sustained inhibition of FLT3 tyrosine kinase activity, with dose escalation limited by toxicities. Widespread hypoxia has been shown by us and others to be prevalent within the confines of the leukemic BM space, and to limit the efficacy of standard chemotherapy (Benito et al., PLoS One 6(8):e23108, 2011). With the goal of exploiting hypoxia as a physiological target and increasing selectivity to FLT3-mutant AML blasts, we have developed SN37169, a hypoxia-activated FLT3 inhibitor pro-drug based on an analogue of the known clinical compound Quizartinib (AC220). Bioreductive trigger conjugation was performed at the final step of the synthesis to provide SN37169 as previously described (Lu et al., Tetrahedron, 69, p9130-9138, 2013). Under hypoxia, SN37169 can undergo an enzymatic one-electron reduction and then fragment selectively to release the cell permeable SN37168 effector, generating higher local concentrations of active FLT3 inhibitor SN37168 and limiting normal tissue exposures.
Pulse radiolysis was first used to determine the electron affinity (E(1)) and rate of fragmentation (kfrag) of SN37169 following one-electron reduction under hypoxia. SN37169 demonstrated E (1) and kfrag parameters ideal for hypoxia-selective cellular metabolism (-449 mV, 62 s-1, respectively).
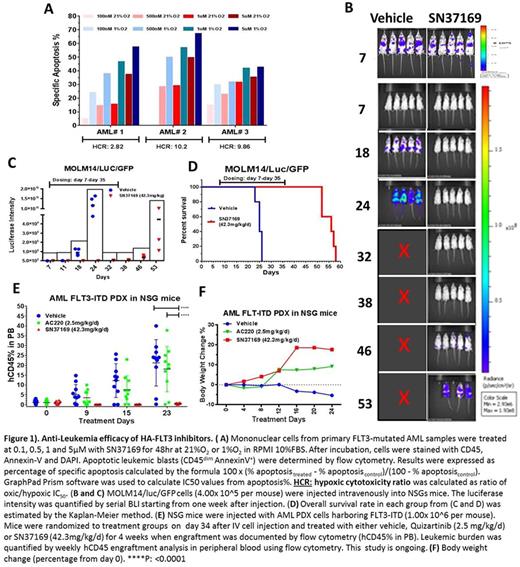
We have previously reported SN37169 to selectively inhibit FLT3 auto phosphorylation in MV4-11 cell line under hypoxic but not oxic conditions, with similar potency to the parental inhibitor (Quizartinib) under normal oxygen culture conditions (Cavazos et al. ASH 2016). We then examined the hypoxia-dependent anti-leukemia activity of SN37169 in 3 primary FLT3-mutated AML samples. SN37169 exhibited hypoxia enhanced apoptosis and growth arrest, with Hypoxia Cytotoxicity Ratios (Oxic/Hypoxic IC50 ratio) of 10.2, 9.9 and 2.8 (Figure1A).
To test the efficacy of SN37169 in vivo, we injected NSGS mice with genetically engineered MOLM14/luc/GFP cells. Mice were randomized to treatment groups on day 7 when engraftment was documented by bioluminescent imaging (BLI), and treated daily with either vehicle or SN37169 at 42.3mg/kg/d IP for 4 weeks. BLI demonstrated 88-99% leukemia reduction during the course of SN37169 treatment compared to vehiclel (Figure 1B and 1C). Overall survival rate in each group was estimated by the Kaplan-Meier method. The median survival for vehicle group was 25 days while for SN37169 group was 56 days (p= 0.0043) (Figure 1D).
To compare the efficacy of SN37169 with that of Quizartinib in vivo, we established an AML FLT3-ITD Patient Derived Xenograft (PDX) in NSG mice. A dose of 2.5 mg/kg/day Quizartinib in mice was estimated to provide a plasma PK profile comparable to 30 mg/day Quizartinib in human subjects based on calculated total AUC exposure across species (Zarrinkar et al., Blood 114:2984-2992, 2009). Weekly human CD45 engraftment analysis in peripheral blood using flow cytometry and daily body weight change measurements has demonstrated a significantly reduced leukemia burden in SN37169 treated group compared to vehicle (p < 0.0001) and Quizartinib (p < 0.0001) treatment groups with no indication of dose related toxicity (Figure 1E and 1F). The efficacy/PD/PK study is ongoing and median survival for all groups is yet to be determined.
In summary, our data indicate hypoxia-enhanced inhibition of mutant FLT3 and its selected downstream signaling, hypoxia-dependent growth arrest and apoptosis in FLT3-mutant AML cells and notable in vivo activity of a novel hypoxia-activated FLT3 inhibitor SN37169. These findings support the functional role of hypoxia in the leukemia microenvironment and implicate potential clinical utility of hypoxia-activated kinase inhibitors with the goal of increasing efficacy and reducing toxicity.
Daver: Pfizer Inc.: Consultancy, Research Funding; Novartis Pharmaceuticals Corporation: Consultancy; Jazz: Consultancy; Incyte Corporation: Honoraria, Research Funding; Sunesis Pharmaceuticals, Inc.: Consultancy, Research Funding; Bristol-Myers Squibb Company: Consultancy, Research Funding; Immunogen: Research Funding; Otsuka America Pharmaceutical, Inc.: Consultancy; Karyopharm: Consultancy, Research Funding; Daiichi-Sankyo: Research Funding; Kiromic: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal